Chemical Nomenclature
•IUPAC (International Union of Pure and Applied Chemistry) is today's most common system for most chemicals.
∟ Ions
∟ Binary Ionic
∟ Polyatomic Ions
∟ Hydrates
∟ Molecular Compounds
∟ Acids / Bases
Chemical Formulas
•Be aware of the differences btwn. ion & compound formulas:
EX: Zn^2+ → Ion charge (Zinc Ion)
BaCl2 → Number of Ions
F^- = Fluoride Ion
N^3- = Nitride Ion
0^2 = Oxide Ion
-Hydrogen (Hydride) = Both metallic & non-metallic
Multivalent Ions
•Some elements can form more than one ion:
EX: Iron → Fe^3+ / Fe^2+
Copper → Cu^2+ / Cu^+
•The top # on the P.T is more common
•IUPAC uses roman numerals in parenthesis to show the charge
•Classical systems uses latin names of elements & suffixes -ic(larger charge) & -ous(smaller charge)
EX: Ferr(ic) Oxide → Fe2O3 (Fe^3+)
Ferr(ous) Oxide → FeO (Fe^2+)
Other Classical Names
•Ferr= Iron •Aunn= Gold
•Cupp= Copper •Plumb= Lead
•Mercur= Mercury •Wolf= Tungsten
•Stann= Tin •Argent= Silver
Hydrates
•Some compounds can form lattices that bond to water molecules.
∟Copper Sulfate ∟Sodium Sulfate
•These crystals contain water inside them which can be released by heating
•To name hydrates
1) Write name of the chemical formula
2) Add a prefix indicating the number of water molecules (mono=1/di=2/tri=3/etc)
3) Add hydrate after the prefix
EX: Cu(SO4)•5H2O(s)
∟Copper(II) Sulphate
∟5 Water
∟pentahydrate
-Paulo Santillan
Monday, 31 October 2011
Thursday, 27 October 2011
Electronic Structure ( Electron Dot Diagrams)
Drawing Electron Dot Diagrams
- The nucleus is represented by the atomic symbol
- For individual elements determine the number of valence electrons
- Electrons are represented by dots around teh symbol
- Four orbitals (one of each side of the nucleus) each holding a maximum of 2e-
- Each orbital gets 1e- before they pair up
Lewis Diagrams for Compounds & Ions
- In covalent compounds electrons are shared
1) Determine the # of valence e- for each atom in the molecule
2) Place atoms so that valence electrons are shared to fill each orbital
Ionic Compounds
- In ionic compounds electrons transfer from one element to another
1) Determine the # of valence electrons on the cation (+)
2) Move these to the anion (-)
3) Draw [ ] around the metal and non-metal
4) Write the Changes
- JanCarlo Paysan
- The nucleus is represented by the atomic symbol
- For individual elements determine the number of valence electrons
- Electrons are represented by dots around teh symbol
- Four orbitals (one of each side of the nucleus) each holding a maximum of 2e-
- Each orbital gets 1e- before they pair up
Lewis Diagrams for Compounds & Ions
- In covalent compounds electrons are shared
1) Determine the # of valence e- for each atom in the molecule
2) Place atoms so that valence electrons are shared to fill each orbital
Ionic Compounds
- In ionic compounds electrons transfer from one element to another
1) Determine the # of valence electrons on the cation (+)
2) Move these to the anion (-)
3) Draw [ ] around the metal and non-metal
4) Write the Changes
- JanCarlo Paysan
Monday, 24 October 2011
Tends on the periodic table
-Elements close to each other on the periodic table displays similar characteristics
-There are 7 important periodic trends:
1. Reactivity
- metals and non-metals show diffrent trends
- the most reactive metal is Francium; the most reactive non metal is Flourine
2. Ion charge
-element ion charge depends on their group name
3. Melting point
1. Elements in the center of the table of the highest melting point
- noble gases have the lowest melting points
- starting from the left and moving right, melting point increases(until middle of the table)
4.Atomic Radius
- radius decrease as you love to the upper right
- Helium has the smallest radius
- Francium has the biggest radius
5. Ionization energy
- ionization energy is the energy needed to completely remove an electron from an atom
- it increases going up and to the right
- all noble gases have high ionization energy
- Helium has the highest ionization energy
- Francium has the lowest ionization energy
- opposite trend from atomic radius
6. Electronegativity
- electronegativity refers to how much atoms want to gain electrons
- follows the same trend as ionization energy
7. Density (not taught during class)
-Paul Dinh
-There are 7 important periodic trends:
1. Reactivity
- metals and non-metals show diffrent trends
- the most reactive metal is Francium; the most reactive non metal is Flourine
2. Ion charge
-element ion charge depends on their group name
3. Melting point
1. Elements in the center of the table of the highest melting point
- noble gases have the lowest melting points
- starting from the left and moving right, melting point increases(until middle of the table)
4.Atomic Radius
- radius decrease as you love to the upper right
- Helium has the smallest radius
- Francium has the biggest radius
5. Ionization energy
- ionization energy is the energy needed to completely remove an electron from an atom
- it increases going up and to the right
- all noble gases have high ionization energy
- Helium has the highest ionization energy
- Francium has the lowest ionization energy
- opposite trend from atomic radius
6. Electronegativity
- electronegativity refers to how much atoms want to gain electrons
- follows the same trend as ionization energy
7. Density (not taught during class)
-Paul Dinh
Sunday, 23 October 2011
Isotopes & Atoms
Atomic number : Number of Protons
Atomic mass - atomic number = # of neutrons
Isotopes - same atomic number but different mass
ex 3 types of hydrogen atoms
1H 2H 3H
(1p)(1p,1n)(1p,2n)
not all atoms of same element are identical
ex
Isotope | Mass # | Atomic # | # of Protons | # of Neutrons
54Fe | 54 | 26 | 26 | 28
66Mn | 56 | 25 | 25 | 31
237Np | 237 | 93 | 93 | 144
14C | 14 | 6 | 6 | 8
Mass Spectrometers
- used to determine the relative abundance and mass of isotopes of elements
(mass spectrometer and how it works)
- JanCarlo Paysan
Atomic mass - atomic number = # of neutrons
Isotopes - same atomic number but different mass
ex 3 types of hydrogen atoms
1H 2H 3H
(1p)(1p,1n)(1p,2n)
not all atoms of same element are identical
ex
Isotope | Mass # | Atomic # | # of Protons | # of Neutrons
54Fe | 54 | 26 | 26 | 28
66Mn | 56 | 25 | 25 | 31
237Np | 237 | 93 | 93 | 144
14C | 14 | 6 | 6 | 8
Mass Spectrometers
- used to determine the relative abundance and mass of isotopes of elements
(mass spectrometer and how it works)
- JanCarlo Paysan
Quantum Mechanics
Bohr Theory
- The electron is a particle that must be in orbital in the atom
Quantum Theory
- The electron is a cloud of negative charge or a wave function
- Orbitals are areas in 3D space where the electrons most probably are
- The energy of the electrons is in its vibrational modes - like notes on a guitar string
- Photons are produced when high energy modes change to lower energy modes
S Orbitals
-each holds 2 electrons
P Orbitals
- There are 3 suborbitals
- Each contains 2 electrons
- Total = 6 electrons
D Orbitals
- There are 5 suborbitals
- Each contains 2 electrons
- Total = 10 electrons
F Orbitals
- There are 7 suborbitals
- Each contains 2 electrons
- Total = 14 electrons
(orbital shapes)
- JanCarlo Paysan
- The electron is a particle that must be in orbital in the atom
Quantum Theory
- The electron is a cloud of negative charge or a wave function
- Orbitals are areas in 3D space where the electrons most probably are
- The energy of the electrons is in its vibrational modes - like notes on a guitar string
- Photons are produced when high energy modes change to lower energy modes
S Orbitals
-each holds 2 electrons
P Orbitals
- There are 3 suborbitals
- Each contains 2 electrons
- Total = 6 electrons
D Orbitals
- There are 5 suborbitals
- Each contains 2 electrons
- Total = 10 electrons
F Orbitals
- There are 7 suborbitals
- Each contains 2 electrons
- Total = 14 electrons
(orbital shapes)
- JanCarlo Paysan
Bohr Diagrams
1) Draw the bohr diagram for the element F
Protons = 9 2e
Atomic Mass = 19 2e
Neutrons = 19-9 = 10 (9p,10n)
2) Draw the bohr diagram for the element Ca
Protons = 20 2e
Atomic Mass = 40.1 8e
Neutrons = 40.1 - 20 = 20.1 8e
2e
(20p,20n)
-Atoms are electrically neutral
-Two different models can be used to describe electron configuration
- energy level model
- Bohr model
-Electrons occupy shells which are divided into orbitals
- 2e in the first orbital
- 8e in the second orbital
- 8e in the third orbital
(examples of bohr diagrams)
- JanCarlo Paysan
Protons = 9 2e
Atomic Mass = 19 2e
Neutrons = 19-9 = 10 (9p,10n)
2) Draw the bohr diagram for the element Ca
Protons = 20 2e
Atomic Mass = 40.1 8e
Neutrons = 40.1 - 20 = 20.1 8e
2e
(20p,20n)
-Atoms are electrically neutral
-Two different models can be used to describe electron configuration
- energy level model
- Bohr model
-Electrons occupy shells which are divided into orbitals
- 2e in the first orbital
- 8e in the second orbital
- 8e in the third orbital
(examples of bohr diagrams)
- JanCarlo Paysan
Sunday, 16 October 2011
Bohr's Diagrams
Bohr (1920)
-Rutherford'd model was inherently unstable
- Protons & electrons should attract eachother
- Matter emits light when it is heated (blackbody radiation)
- Light travels as photons
- the energy photons carry depends on their wavelength
Separate the white light into colours with a diffraction granting or prism
Each line represents a photon of light emitted from the excited atom
These are unique sets of lines for each element
-Bohr based his model on the energy (light) emitted by different atoms
- Each atom has a specific spectrum of light
Examples of different atom spectrums of light:
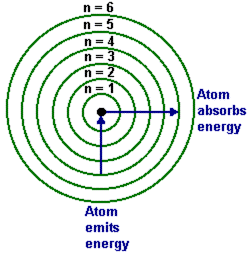
Bohr's theory
- Electrons exist in orbitals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light
- JanCarlo Paysan
-Rutherford'd model was inherently unstable
- Protons & electrons should attract eachother
- Matter emits light when it is heated (blackbody radiation)
- Light travels as photons
- the energy photons carry depends on their wavelength
Separate the white light into colours with a diffraction granting or prism
Each line represents a photon of light emitted from the excited atom
These are unique sets of lines for each element
-Bohr based his model on the energy (light) emitted by different atoms
- Each atom has a specific spectrum of light
Examples of different atom spectrums of light:
Bohr's theory
- Electrons exist in orbitals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light
- JanCarlo Paysan
Density & Graphing
Density
- The density of an object is its mass divided by its volume
- d=m/v
- Usually expressed in : kg/L, kg/m^3, kg/m^2
- Examples : d= 135kg / 65L = 2.1kg/L
d= 54kg / 27m^3 = 2kg/m^3
d= 1200kg / 51m^2 =24kg/m^2
Graphing
*All graphs must contain 5 important things*
1) Labelled Axis
2) Appropriate Scale
3) Title
4) Data Points
5) Line of Best Fit
Example of proper graph :
- Three things can be done when working with graphs
1) Reading the graphs
2) Find the slope (rise/run)
3) Find the area under the graph
- Linear Graph :
- Inverse Graph :
- JanCarlo Paysan
- The density of an object is its mass divided by its volume
- d=m/v
- Usually expressed in : kg/L, kg/m^3, kg/m^2
- Examples : d= 135kg / 65L = 2.1kg/L
d= 54kg / 27m^3 = 2kg/m^3
d= 1200kg / 51m^2 =24kg/m^2
Graphing
*All graphs must contain 5 important things*
1) Labelled Axis
2) Appropriate Scale
3) Title
4) Data Points
5) Line of Best Fit
Example of proper graph :
- Three things can be done when working with graphs
1) Reading the graphs
2) Find the slope (rise/run)
3) Find the area under the graph
- Linear Graph :
- Inverse Graph :
- JanCarlo Paysan
Subscribe to:
Comments (Atom)