1) Draw the bohr diagram for the element F
Protons = 9 2e
Atomic Mass = 19 2e
Neutrons = 19-9 = 10 (9p,10n)
2) Draw the bohr diagram for the element Ca
Protons = 20 2e
Atomic Mass = 40.1 8e
Neutrons = 40.1 - 20 = 20.1 8e
2e
(20p,20n)
-Atoms are electrically neutral
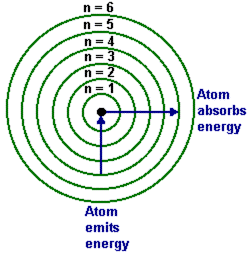
-Two different models can be used to describe electron configuration
- energy level model
- Bohr model
-Electrons occupy shells which are divided into orbitals
- 2e in the first orbital
- 8e in the second orbital
- 8e in the third orbital
(examples of bohr diagrams)
- JanCarlo Paysan

No comments:
Post a Comment