- At a specific pressure and temperature one more of every gas has the same volume
- At 0 degrees celsius and 101.3kPa 1mol = 22.4L
- This Temperature and pressure is called STP
- 22.4L/mol is the molar volume at STP
ex
2.5mol x 22.4L = 56L
1 mol
11.6L x 1 mol = 0.518mol
22.4L
11.5mol x 22.4L = 258L
1 mol
1cc = 1mL
1cm^3 = 1mL
- JanCarlo Paysan
Saturday, 19 November 2011
Friday, 18 November 2011
converting from moles to molecules
converting 2 moles of Au
like converting distance:
2 mol x 6.02x10^23
1 mol
the mole's cancel each other out so you get
2 x 6.02x10^23 = 12.01x10^23 = 12.0x10^23
1
you multiply and then convert to significant digits.
-Paul Dinh
like converting distance:
2 mol x 6.02x10^23
1 mol
the mole's cancel each other out so you get
2 x 6.02x10^23 = 12.01x10^23 = 12.0x10^23
1
you multiply and then convert to significant digits.
-Paul Dinh
Tuesday, 15 November 2011
Molar Mass (Mass of Atoms)
•The mass (in grams) of 1 mole of a substance is called the molar mass.
•It can be determined from the atomic mass on the periodic table
•Measured in g/mol
*Molar Mass* -The atomic weight of an element expressed in grams is the mass of one mole of the element.
Molar Mass of Compounds
•To determine the molar mass of a compound add the mass of all the atoms together.
-Paulo Santillan
•It can be determined from the atomic mass on the periodic table
•Measured in g/mol
*Molar Mass* -The atomic weight of an element expressed in grams is the mass of one mole of the element.
Molar Mass of Compounds
•To determine the molar mass of a compound add the mass of all the atoms together.
-Paulo Santillan
Avrogado's Number (How we count atoms)
•Atoms & molecules are extremely small
•Macroscopic objects contain too many to count or weigh individually
•Amedeo Avrogado proposed that the # of atoms 12.00000g of Carbon be equal to a constant (This is equal to 1 mol of Carbon)
•This value is now called Avrogado's Number & froms the basis of all quantitative chemistry.
• Avrogado's Number → 1mol = 6.02x10^23
•One mole is simply a multiple of things, such as:
∟1 pair = 2
∟1 dozen = 12
∟1 century = 100
∟1 mol = 6.02x10^23
•One mole represents a huge # of particles:
Particle
•Atoms Element 6.02x10^23 Fe
1 mol
•Molecules Covalent Compound 6.02x10^23 CO2
1 mol
•Formula Unit Ionic Compound 6.02x10^23 NaCl
1 mol
_____________________________________________________________________________
-Paulo Santillan
•Macroscopic objects contain too many to count or weigh individually
•Amedeo Avrogado proposed that the # of atoms 12.00000g of Carbon be equal to a constant (This is equal to 1 mol of Carbon)
•This value is now called Avrogado's Number & froms the basis of all quantitative chemistry.
• Avrogado's Number → 1mol = 6.02x10^23
•One mole is simply a multiple of things, such as:
∟1 pair = 2
∟1 dozen = 12
∟1 century = 100
∟1 mol = 6.02x10^23
•One mole represents a huge # of particles:
Particle
•Atoms Element 6.02x10^23 Fe
1 mol
•Molecules Covalent Compound 6.02x10^23 CO2
1 mol
•Formula Unit Ionic Compound 6.02x10^23 NaCl
1 mol
_____________________________________________________________________________
-Paulo Santillan
Sunday, 6 November 2011
Chem Lab
in lab we did we had to pick a hydrate and follow the steps
1) we had to weight the empty dry test tube
2) we had to weight the test tube with the hydrate in it
3) we had to light the bunsen burner
4) we had to boil all the water out of the hydrate
5) once we boiled all the water out we had to weigh the hydrate again
6) we calculated how much grams of water was boiled off and how much percent of water the hydrate was
7) we had to calculate our percent error for the percentage of water boiled off
- JanCarlo Paysan
1) we had to weight the empty dry test tube
2) we had to weight the test tube with the hydrate in it
3) we had to light the bunsen burner
4) we had to boil all the water out of the hydrate
5) once we boiled all the water out we had to weigh the hydrate again
6) we calculated how much grams of water was boiled off and how much percent of water the hydrate was
7) we had to calculate our percent error for the percentage of water boiled off
- JanCarlo Paysan
Monday, 31 October 2011
Naming Compunds
Chemical Nomenclature
•IUPAC (International Union of Pure and Applied Chemistry) is today's most common system for most chemicals.
∟ Ions
∟ Binary Ionic
∟ Polyatomic Ions
∟ Hydrates
∟ Molecular Compounds
∟ Acids / Bases
Chemical Formulas
•Be aware of the differences btwn. ion & compound formulas:
EX: Zn^2+ → Ion charge (Zinc Ion)
BaCl2 → Number of Ions
F^- = Fluoride Ion
N^3- = Nitride Ion
0^2 = Oxide Ion
-Hydrogen (Hydride) = Both metallic & non-metallic
Multivalent Ions
•Some elements can form more than one ion:
EX: Iron → Fe^3+ / Fe^2+
Copper → Cu^2+ / Cu^+
•The top # on the P.T is more common
•IUPAC uses roman numerals in parenthesis to show the charge
•Classical systems uses latin names of elements & suffixes -ic(larger charge) & -ous(smaller charge)
EX: Ferr(ic) Oxide → Fe2O3 (Fe^3+)
Ferr(ous) Oxide → FeO (Fe^2+)
Other Classical Names
•Ferr= Iron •Aunn= Gold
•Cupp= Copper •Plumb= Lead
•Mercur= Mercury •Wolf= Tungsten
•Stann= Tin •Argent= Silver
Hydrates
•Some compounds can form lattices that bond to water molecules.
∟Copper Sulfate ∟Sodium Sulfate
•These crystals contain water inside them which can be released by heating
•To name hydrates
1) Write name of the chemical formula
2) Add a prefix indicating the number of water molecules (mono=1/di=2/tri=3/etc)
3) Add hydrate after the prefix
EX: Cu(SO4)•5H2O(s)
∟Copper(II) Sulphate
∟5 Water
∟pentahydrate
-Paulo Santillan
•IUPAC (International Union of Pure and Applied Chemistry) is today's most common system for most chemicals.
∟ Ions
∟ Binary Ionic
∟ Polyatomic Ions
∟ Hydrates
∟ Molecular Compounds
∟ Acids / Bases
Chemical Formulas
•Be aware of the differences btwn. ion & compound formulas:
EX: Zn^2+ → Ion charge (Zinc Ion)
BaCl2 → Number of Ions
F^- = Fluoride Ion
N^3- = Nitride Ion
0^2 = Oxide Ion
-Hydrogen (Hydride) = Both metallic & non-metallic
Multivalent Ions
•Some elements can form more than one ion:
EX: Iron → Fe^3+ / Fe^2+
Copper → Cu^2+ / Cu^+
•The top # on the P.T is more common
•IUPAC uses roman numerals in parenthesis to show the charge
•Classical systems uses latin names of elements & suffixes -ic(larger charge) & -ous(smaller charge)
EX: Ferr(ic) Oxide → Fe2O3 (Fe^3+)
Ferr(ous) Oxide → FeO (Fe^2+)
Other Classical Names
•Ferr= Iron •Aunn= Gold
•Cupp= Copper •Plumb= Lead
•Mercur= Mercury •Wolf= Tungsten
•Stann= Tin •Argent= Silver
Hydrates
•Some compounds can form lattices that bond to water molecules.
∟Copper Sulfate ∟Sodium Sulfate
•These crystals contain water inside them which can be released by heating
•To name hydrates
1) Write name of the chemical formula
2) Add a prefix indicating the number of water molecules (mono=1/di=2/tri=3/etc)
3) Add hydrate after the prefix
EX: Cu(SO4)•5H2O(s)
∟Copper(II) Sulphate
∟5 Water
∟pentahydrate
-Paulo Santillan
Thursday, 27 October 2011
Electronic Structure ( Electron Dot Diagrams)
Drawing Electron Dot Diagrams
- The nucleus is represented by the atomic symbol
- For individual elements determine the number of valence electrons
- Electrons are represented by dots around teh symbol
- Four orbitals (one of each side of the nucleus) each holding a maximum of 2e-
- Each orbital gets 1e- before they pair up
Lewis Diagrams for Compounds & Ions
- In covalent compounds electrons are shared
1) Determine the # of valence e- for each atom in the molecule
2) Place atoms so that valence electrons are shared to fill each orbital
Ionic Compounds
- In ionic compounds electrons transfer from one element to another
1) Determine the # of valence electrons on the cation (+)
2) Move these to the anion (-)
3) Draw [ ] around the metal and non-metal
4) Write the Changes
- JanCarlo Paysan
- The nucleus is represented by the atomic symbol
- For individual elements determine the number of valence electrons
- Electrons are represented by dots around teh symbol
- Four orbitals (one of each side of the nucleus) each holding a maximum of 2e-
- Each orbital gets 1e- before they pair up
Lewis Diagrams for Compounds & Ions
- In covalent compounds electrons are shared
1) Determine the # of valence e- for each atom in the molecule
2) Place atoms so that valence electrons are shared to fill each orbital
Ionic Compounds
- In ionic compounds electrons transfer from one element to another
1) Determine the # of valence electrons on the cation (+)
2) Move these to the anion (-)
3) Draw [ ] around the metal and non-metal
4) Write the Changes
- JanCarlo Paysan
Monday, 24 October 2011
Tends on the periodic table
-Elements close to each other on the periodic table displays similar characteristics
-There are 7 important periodic trends:
1. Reactivity
- metals and non-metals show diffrent trends
- the most reactive metal is Francium; the most reactive non metal is Flourine
2. Ion charge
-element ion charge depends on their group name
3. Melting point
1. Elements in the center of the table of the highest melting point
- noble gases have the lowest melting points
- starting from the left and moving right, melting point increases(until middle of the table)
4.Atomic Radius
- radius decrease as you love to the upper right
- Helium has the smallest radius
- Francium has the biggest radius
5. Ionization energy
- ionization energy is the energy needed to completely remove an electron from an atom
- it increases going up and to the right
- all noble gases have high ionization energy
- Helium has the highest ionization energy
- Francium has the lowest ionization energy
- opposite trend from atomic radius
6. Electronegativity
- electronegativity refers to how much atoms want to gain electrons
- follows the same trend as ionization energy
7. Density (not taught during class)
-Paul Dinh
-There are 7 important periodic trends:
1. Reactivity
- metals and non-metals show diffrent trends
- the most reactive metal is Francium; the most reactive non metal is Flourine
2. Ion charge
-element ion charge depends on their group name
3. Melting point
1. Elements in the center of the table of the highest melting point
- noble gases have the lowest melting points
- starting from the left and moving right, melting point increases(until middle of the table)
4.Atomic Radius
- radius decrease as you love to the upper right
- Helium has the smallest radius
- Francium has the biggest radius
5. Ionization energy
- ionization energy is the energy needed to completely remove an electron from an atom
- it increases going up and to the right
- all noble gases have high ionization energy
- Helium has the highest ionization energy
- Francium has the lowest ionization energy
- opposite trend from atomic radius
6. Electronegativity
- electronegativity refers to how much atoms want to gain electrons
- follows the same trend as ionization energy
7. Density (not taught during class)
-Paul Dinh
Sunday, 23 October 2011
Isotopes & Atoms
Atomic number : Number of Protons
Atomic mass - atomic number = # of neutrons
Isotopes - same atomic number but different mass
ex 3 types of hydrogen atoms
1H 2H 3H
(1p)(1p,1n)(1p,2n)
not all atoms of same element are identical
ex
Isotope | Mass # | Atomic # | # of Protons | # of Neutrons
54Fe | 54 | 26 | 26 | 28
66Mn | 56 | 25 | 25 | 31
237Np | 237 | 93 | 93 | 144
14C | 14 | 6 | 6 | 8
Mass Spectrometers
- used to determine the relative abundance and mass of isotopes of elements
(mass spectrometer and how it works)
- JanCarlo Paysan
Atomic mass - atomic number = # of neutrons
Isotopes - same atomic number but different mass
ex 3 types of hydrogen atoms
1H 2H 3H
(1p)(1p,1n)(1p,2n)
not all atoms of same element are identical
ex
Isotope | Mass # | Atomic # | # of Protons | # of Neutrons
54Fe | 54 | 26 | 26 | 28
66Mn | 56 | 25 | 25 | 31
237Np | 237 | 93 | 93 | 144
14C | 14 | 6 | 6 | 8
Mass Spectrometers
- used to determine the relative abundance and mass of isotopes of elements
(mass spectrometer and how it works)
- JanCarlo Paysan
Quantum Mechanics
Bohr Theory
- The electron is a particle that must be in orbital in the atom
Quantum Theory
- The electron is a cloud of negative charge or a wave function
- Orbitals are areas in 3D space where the electrons most probably are
- The energy of the electrons is in its vibrational modes - like notes on a guitar string
- Photons are produced when high energy modes change to lower energy modes
S Orbitals
-each holds 2 electrons
P Orbitals
- There are 3 suborbitals
- Each contains 2 electrons
- Total = 6 electrons
D Orbitals
- There are 5 suborbitals
- Each contains 2 electrons
- Total = 10 electrons
F Orbitals
- There are 7 suborbitals
- Each contains 2 electrons
- Total = 14 electrons
(orbital shapes)
- JanCarlo Paysan
- The electron is a particle that must be in orbital in the atom
Quantum Theory
- The electron is a cloud of negative charge or a wave function
- Orbitals are areas in 3D space where the electrons most probably are
- The energy of the electrons is in its vibrational modes - like notes on a guitar string
- Photons are produced when high energy modes change to lower energy modes
S Orbitals
-each holds 2 electrons
P Orbitals
- There are 3 suborbitals
- Each contains 2 electrons
- Total = 6 electrons
D Orbitals
- There are 5 suborbitals
- Each contains 2 electrons
- Total = 10 electrons
F Orbitals
- There are 7 suborbitals
- Each contains 2 electrons
- Total = 14 electrons
(orbital shapes)
- JanCarlo Paysan
Bohr Diagrams
1) Draw the bohr diagram for the element F
Protons = 9 2e
Atomic Mass = 19 2e
Neutrons = 19-9 = 10 (9p,10n)
2) Draw the bohr diagram for the element Ca
Protons = 20 2e
Atomic Mass = 40.1 8e
Neutrons = 40.1 - 20 = 20.1 8e
2e
(20p,20n)
-Atoms are electrically neutral
-Two different models can be used to describe electron configuration
- energy level model
- Bohr model
-Electrons occupy shells which are divided into orbitals
- 2e in the first orbital
- 8e in the second orbital
- 8e in the third orbital
(examples of bohr diagrams)
- JanCarlo Paysan
Protons = 9 2e
Atomic Mass = 19 2e
Neutrons = 19-9 = 10 (9p,10n)
2) Draw the bohr diagram for the element Ca
Protons = 20 2e
Atomic Mass = 40.1 8e
Neutrons = 40.1 - 20 = 20.1 8e
2e
(20p,20n)
-Atoms are electrically neutral
-Two different models can be used to describe electron configuration
- energy level model
- Bohr model
-Electrons occupy shells which are divided into orbitals
- 2e in the first orbital
- 8e in the second orbital
- 8e in the third orbital
(examples of bohr diagrams)
- JanCarlo Paysan
Sunday, 16 October 2011
Bohr's Diagrams
Bohr (1920)
-Rutherford'd model was inherently unstable
- Protons & electrons should attract eachother
- Matter emits light when it is heated (blackbody radiation)
- Light travels as photons
- the energy photons carry depends on their wavelength
Separate the white light into colours with a diffraction granting or prism
Each line represents a photon of light emitted from the excited atom
These are unique sets of lines for each element
-Bohr based his model on the energy (light) emitted by different atoms
- Each atom has a specific spectrum of light
Examples of different atom spectrums of light:
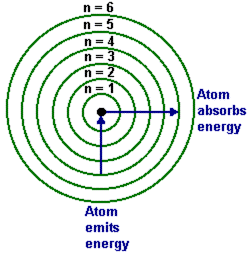
Bohr's theory
- Electrons exist in orbitals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light
- JanCarlo Paysan
-Rutherford'd model was inherently unstable
- Protons & electrons should attract eachother
- Matter emits light when it is heated (blackbody radiation)
- Light travels as photons
- the energy photons carry depends on their wavelength
Separate the white light into colours with a diffraction granting or prism
Each line represents a photon of light emitted from the excited atom
These are unique sets of lines for each element
-Bohr based his model on the energy (light) emitted by different atoms
- Each atom has a specific spectrum of light
Examples of different atom spectrums of light:
Bohr's theory
- Electrons exist in orbitals
- When they absorb energy they move to a higher orbital
- As they fall from a higher orbital to a lower one they release energy as a photon of light
- JanCarlo Paysan
Density & Graphing
Density
- The density of an object is its mass divided by its volume
- d=m/v
- Usually expressed in : kg/L, kg/m^3, kg/m^2
- Examples : d= 135kg / 65L = 2.1kg/L
d= 54kg / 27m^3 = 2kg/m^3
d= 1200kg / 51m^2 =24kg/m^2
Graphing
*All graphs must contain 5 important things*
1) Labelled Axis
2) Appropriate Scale
3) Title
4) Data Points
5) Line of Best Fit
Example of proper graph :
- Three things can be done when working with graphs
1) Reading the graphs
2) Find the slope (rise/run)
3) Find the area under the graph
- Linear Graph :
- Inverse Graph :
- JanCarlo Paysan
- The density of an object is its mass divided by its volume
- d=m/v
- Usually expressed in : kg/L, kg/m^3, kg/m^2
- Examples : d= 135kg / 65L = 2.1kg/L
d= 54kg / 27m^3 = 2kg/m^3
d= 1200kg / 51m^2 =24kg/m^2
Graphing
*All graphs must contain 5 important things*
1) Labelled Axis
2) Appropriate Scale
3) Title
4) Data Points
5) Line of Best Fit
Example of proper graph :
- Three things can be done when working with graphs
1) Reading the graphs
2) Find the slope (rise/run)
3) Find the area under the graph
- Linear Graph :
- Inverse Graph :
- JanCarlo Paysan
Wednesday, 28 September 2011
Unit and Dimensional Analysis
4 steps how to convert units
1. Identify Solution
2.Find Conversion Factors
3. Place units in the right order and place
4. Cancel out the units
ex:
You want to convert 35 € to Canadian currency
To convert distance over time:
You want to change kilometers per hour to meters per second
It is crucial to learn how to convert units correctly in science, for more examples, click here
- Paul
1. Identify Solution
2.Find Conversion Factors
3. Place units in the right order and place
4. Cancel out the units
ex:
You want to convert 35 € to Canadian currency
To convert distance over time:
You want to change kilometers per hour to meters per second
It is crucial to learn how to convert units correctly in science, for more examples, click here
- Paul
Sunday, 25 September 2011
Significant Digits & Scientific Notation
Significant Digits
The certain digits and the estimated digit of a measurement are together
Not all digits are significant
-All numbers other than zero are significant
-Place saving zeros aren't significant (decimals)
∟0.005= 1 S.D. ∟0.0050= 2 S.D.
When multiplying and dividing:
-round your answer to the lowest number of S.D in the initial numbers
When adding and subtracting:
-round to the lowest decimal place from the original numbers
Scientific Notation
When writing in scientific notation, numbers split into 2 parts:
-Number btwn. 1→10
-Power of ten
∟1000 = 10 x 10 x 10 = 10^3
-Paulo Santillan
The certain digits and the estimated digit of a measurement are together
Not all digits are significant
-All numbers other than zero are significant
-Place saving zeros aren't significant (decimals)
∟
When multiplying and dividing:
-round your answer to the lowest number of S.D in the initial numbers
When adding and subtracting:
-round to the lowest decimal place from the original numbers
Scientific Notation
When writing in scientific notation, numbers split into 2 parts:
-Number btwn. 1→10
-Power of ten
∟1000 = 10 x 10 x 10 = 10^3
-Paulo Santillan
Thursday, 22 September 2011
Measurement and Error
SI Unit Prefixes, Multiples, and Symbols:
SI Base Units:
Base unit: A fundamental unit that is defined arbitrarily and not by combinations of other units. The base units of the SI system are the meter, kilogram, second, ampere, kelvin, mole, and candela.
Derived Units:
Derived Unit: combinations of base units used to measure area, volume, force, pressure, energy, power, voltage, frequency, and electric current.
Error
- Error is a fundamental part of science
- There are usually 3 reasons for error
- Physical errors in the measuring device
- "sloppy" measuring
- Changing factors
- 2 ways to calculate Error
- Absolute Error
- Formula : Absolute Error = Measured Value - Accepted Value
- Percent Error
- Formula :
- JanCarlo Paysan
SI Base Units:
Base unit: A fundamental unit that is defined arbitrarily and not by combinations of other units. The base units of the SI system are the meter, kilogram, second, ampere, kelvin, mole, and candela.
Derived Units:
Derived Unit: combinations of base units used to measure area, volume, force, pressure, energy, power, voltage, frequency, and electric current.
Error
- Error is a fundamental part of science
- There are usually 3 reasons for error
- Physical errors in the measuring device
- "sloppy" measuring
- Changing factors
- 2 ways to calculate Error
- Absolute Error
- Formula : Absolute Error = Measured Value - Accepted Value
- Percent Error
- Formula :
- JanCarlo Paysan
Wednesday, 21 September 2011
Chemistry Units Joke
Q: What is the world's most smallest animal?
A: A "nano"-mole
-Paulo Santillan :D
A: A "nano"-mole
-Paulo Santillan :D
Tuesday, 20 September 2011
Classification of Chemicals
Understanding matter begins w/ how we name it. We can divide matter into 2 types:
-Homogeneous: Consists of only one visible compound
-ex: distilled water, oxygen, graphite
-Heterogeneous: Contains more than one visible component
-ex: chocolate chip cookie, granite
Pure Substances
There are 2 types of Pure Substances:
-Elements: Substances that cannot be broken down into simpler substances by chemical reactions
-ex: oxygen, iron, magnesium
-Compounds: Substances that are made up of 2/more elements and can be changed into elements (or other compounds) by chemical reactions
-water(H2O), sugar(C12H22O11)
H2O + energy → (H2) + (O2)
∟ compounds split apart(by adding energy)
Telling the Difference
To identify an element or compound, the difference is only "visible" on the atomic level
One method is to connect the substance to an electric current.
-(Electrolysis) can split the compound apart into its constituent elements
Solution
A solution is a homogeneous mixture of 2/more substances
-Usually involve liquids (not always, like fog & steel)
The component present in greater amount is the solvent
-Water is most common solvent
-The symbol (aq) is used when something is dissolved
The component present in smaller amount is the solute
Mixtures
Mixtures can be easily identified, or can be confused as pure substances
-Heterogeneous mixtures, different parts clearly visible (granite, sand fog)
-Homogeneous mixtures, different parts aren't visible (salt water, air, brass)
Separating Mixtures
Many methods to separate mixtures, depending on type:
-By hand
-Filtration*
-Distillation*
-Crystallization
-Chromatography
^All physical changes
*Heterogeneous mixtures only
-Paulo Santillan
-Homogeneous: Consists of only one visible compound
-ex: distilled water, oxygen, graphite
-Heterogeneous: Contains more than one visible component
-ex: chocolate chip cookie, granite
Pure Substances
There are 2 types of Pure Substances:
-Elements: Substances that cannot be broken down into simpler substances by chemical reactions
-ex: oxygen, iron, magnesium
-Compounds: Substances that are made up of 2/more elements and can be changed into elements (or other compounds) by chemical reactions
-water(H2O), sugar(C12H22O11)
H2O + energy → (H2) + (O2)
∟ compounds split apart(by adding energy)
Telling the Difference
To identify an element or compound, the difference is only "visible" on the atomic level
One method is to connect the substance to an electric current.
-(Electrolysis) can split the compound apart into its constituent elements
Solution
A solution is a homogeneous mixture of 2/more substances
-Usually involve liquids (not always, like fog & steel)
The component present in greater amount is the solvent
-Water is most common solvent
-The symbol (aq) is used when something is dissolved
The component present in smaller amount is the solute
Mixtures
Mixtures can be easily identified, or can be confused as pure substances
-Heterogeneous mixtures, different parts clearly visible (granite, sand fog)
-Homogeneous mixtures, different parts aren't visible (salt water, air, brass)
Separating Mixtures
Many methods to separate mixtures, depending on type:
-By hand
-Filtration*
-Distillation*
-Crystallization
-Chromatography
^All physical changes
*Heterogeneous mixtures only
-Paulo Santillan
Sunday, 18 September 2011
Physical and Chemical Change pt. 2
Today we examined the chemical reaction when lead nitrate and pottasium iodide was mixed together to form a yellow dye. We observed little crystal like balls accumulate resulting from the mix.
-Paul
-Paul
Physical & Chemical Changes
Matter can undergo many changes
3 Categories
- Physical Changes
- Chemical Changes
- Nuclear Changes
Physical Changes
- Involves changing shape or state of matter
- ex. crushing, tearing, ect....
- No new substances are formed
- ex. boiling water, cutting wood, smashing cars, etc..
Phase Changets
- Changing from a solid to a gas can often be confused as a chemical change
- chemicals remain the same
During the melting process chemicals usually follow this path
Chemical Change
- New substances are form
-Properties of the matter change
-conductivity, acidity, colour, etc..
-ex. iron rusting, burning wood, digesting food
Changes in Physical State
- Solid to Gas = Sublimation
- Solid to Liquid = Melting
- Liquid to Solid = Solidification / Freezing
- Liquid to Gas = Evaporation
- Gas to Liquid = Condensation
- Gas to Solid = Deposition / Sublimation
- JanCarlo Paysan
3 Categories
- Physical Changes
- Chemical Changes
- Nuclear Changes
Physical Changes
- Involves changing shape or state of matter
- ex. crushing, tearing, ect....
- No new substances are formed
- ex. boiling water, cutting wood, smashing cars, etc..
Phase Changets
- Changing from a solid to a gas can often be confused as a chemical change
- chemicals remain the same
During the melting process chemicals usually follow this path
Chemical Change
- New substances are form
-Properties of the matter change
-conductivity, acidity, colour, etc..
-ex. iron rusting, burning wood, digesting food
Changes in Physical State
- Solid to Gas = Sublimation
- Solid to Liquid = Melting
- Liquid to Solid = Solidification / Freezing
- Liquid to Gas = Evaporation
- Gas to Liquid = Condensation
- Gas to Solid = Deposition / Sublimation
- JanCarlo Paysan
Tuesday, 13 September 2011
Blancing and Word Equations
*Phase symbols are subscripts that indicate the phase of the chemical.
ex. Al(s), H2O(l), H2(g), AgNO3(aq)
*Diatomic Molecules
Example of a Word Equation to a Balanced Equation
Word Equation : "a solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate"
Balanced Equation: Ba3(PO4)2(aq) + 3Na2SO4(aq) ---> 3BaSO4(s) + 2Na3PO4(aq)
ex. Al(s), H2O(l), H2(g), AgNO3(aq)
- (s) -----> Solid
- (l) ------> Liquid
- (g)-----> Gas
- (aq)----> Aqueous
*Diatomic Molecules
- H2 Hydrogen *Polyatomic Molecules*
- O2 Oxygen - P4 Phosphorus
- F2 Fluorine - S8 Sulphur
- Br2 Bromine
- I2 Iodine
- N2 Nitrogen
- Cl2 Chlorine
Example of a Word Equation to a Balanced Equation
Word Equation : "a solution of barium phosphate is mixed with aqueous sodium sulphate to yield solid barium sulphate and aqueous sodium phosphate"
Balanced Equation: Ba3(PO4)2(aq) + 3Na2SO4(aq) ---> 3BaSO4(s) + 2Na3PO4(aq)
Monday, 12 September 2011
Safety Rules in a Lab
1) Work "only" under teacher supervision
2) Follow your Teacher's directions
3) Read all instructions and procedures
4) Notify your Teacher of any problems
5) Know how to use the Safety Equipment and materials in the Laboratory
6)Wear approved Safety Goggles and Lab coat
7)Wear proper shoes (no open toes)
8) Tie your hair back if you have long hair
9) Don't leave any unnecessary things out in the open, place them under a table (ex: bags)
16) Throw away any broken glass
17) If on fire or any chemicals get on you, make sure you know how to use the shower head/ eye wash
18) Read chemical labels on glass
19)Don't taste anything
20) Food, drinks, and gum are prohibited
21) Do not look directly at test tubes
22) Don't directly smell chemicals, waft a small scent only
23) Clean up Afterward
-Paulo Santillan
2) Follow your Teacher's directions
3) Read all instructions and procedures
4) Notify your Teacher of any problems
5) Know how to use the Safety Equipment and materials in the Laboratory
6)Wear approved Safety Goggles and Lab coat
7)Wear proper shoes (no open toes)
8) Tie your hair back if you have long hair
9) Don't leave any unnecessary things out in the open, place them under a table (ex: bags)
10)Avoid making any awkward transfers with the chemicals used
11)"If it's Hot, Let it Cool"
12)Carry chemicals with extreme caution
13) No fooling around! (especially during experiments)
14)Dispose of chemical wastes properly
15) Make sure all equipment used during a lab is safe and "usable"16) Throw away any broken glass
17) If on fire or any chemicals get on you, make sure you know how to use the shower head/ eye wash
18) Read chemical labels on glass
19)Don't taste anything
20) Food, drinks, and gum are prohibited
21) Do not look directly at test tubes
22) Don't directly smell chemicals, waft a small scent only
23) Clean up Afterward
-Paulo Santillan
Subscribe to:
Comments (Atom)